Characteristics of EM Radiation
After reading this section you will be able to do the following:
- Explain what the electromagnetic spectrum is and how scientists use it.
So far we have learned about the atom, electricity, and magnetism,and have touched on electromagnetism. Two key points to remember about the characteristics of EM radiation (e.g., microwaves, X-rays, and gamma rays) are that EM radiation is not bits of matter, they are electromagnetic wave forms possessing no charge and no mass, and they can be characterized by frequency, wavelength, and velocity. Let's take a closer look at the characteristics of these wave forms so that we may better understand the nature of them.
Electromagnetic (EM) Waves
X- and gamma rays are part of what scientists refer to as the electromagnetic (EM) spectrum. They are waveforms that are part of a family in which some of the relatives are very familiar to us, such as light rays, infrared heat rays, and radio waves. However, X- and gamma rays cannot been seen, felt, or heard. In other words, our normal senses cannot detect them. Since X- and gamma rays have no mass and no electrical charge, they are not influenced by electrical and magnetic fields and will travel in straight lines.
Radiation possesses a dual character. Acting somewhat like a particle at times and like a wave at other times. The name that has been given to the small "packets" of energy with these characteristics is "photon." It is said that the radiation photon is a wave that is both electric and magnetic in nature. Electromagnetic radiation has also been described in terms of a stream of photons (massless particles) each traveling in a wave-like pattern and moving at the speed of light.
This diagram shows the electromagnetic spectrum. Notice the changes in wavelengths of the various wave forms.
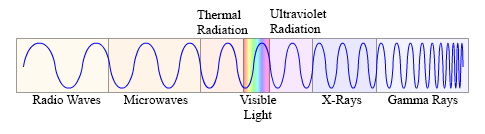

Every point across the spectrum represents a wave form of differing wavelength. It should be noted that the lines between the groupings are not precise, and that each group phases into the next.
Wave forms may be graphically represented as following:

Wavelength
Just as with mechanical waves on the previous page, the distance between the peaks or the distance between the troughs of the waves is the length of one wave, or more precisely the wavelength. Note that the electromagnetic waves vary in length from one end of the spectrum to the other. Some radio waves may be several miles longs, while X and gamma rays are measured in angstroms, and fractions of angstrom. An angstrom unit is equivalent to 0.00000001 centimeters (100 millionths cm). If you want to compare this to an inch, a centimeter is equal to 0.394 inch. You can see that the wavelength as measured in angstrom units is extremely small.
Frequency
Another term used to describe a wave is frequency. Since waves are moving, we define frequency as the number of waves that pass a given point in a specified unit of time. The unit commonly used is Hertz which is the number of wave cycles pass a point in one second. So one cycle per second equals one Hertz. . The frequency of a wave is indirectly proportional to the wavelength of the radiation. This means that as one value goes up, the other goes down. You can see that as the wavelength increases, it takes longer for one complete wave to pass a point and, therefore, the frequency goes down.
Frequency vs. Velocity
Don't confuse the frequency of a wave with the speed of travel or velocity of a wave; they are not the same or even related. X and gamma rays and all other members of the electromagnetic spectrum, travel at the same speed, 186,000 miles per second (3x10^8 meters per second). This speed is known as the speed of light, or actually the speed of electromagnetic radiation. Frequency describes how many complete wave cycles go flying by in one second.
Let's pause for a quick check of understanding
Now would be a good time to apply what we have learned about the characteristics of electromagnetic waves. Lets look at some specific examples and see if we understand.
Here are two wave forms (wave A, and wave B) with different wavelength. Which one has the greater wavelength and which one has the greater frequency? If wave A has a frequency of 1 Hertz, what is the frequency of wave B?

You should have concluded that Wave A has a longer wavelength; therefore it will have a lower frequency than Wave B. Remember wavelength and frequency is inversely proportional; meaning that as one increases, the other decreases by a proportional amount. You should have also concluded that Wave B has a frequency of 2 Hertz. For every complete cycle of Wave A, Wave B completes 2 cycles.
Importance of Wavelength and Frequency
Not only are the wavelength and frequency of the wave linked, but they are also linked to the amount of energy of the wave. If two waves have that same amplitude then they each have the same amount of energy in one complete a cycle. Since waves with short wavelengths complete more cycles per second, you can see that they can transfer more energy than waves with longer wavelengths can in the same amount of time. Therefore, shorter wavelengths and higher frequency equates to more energy. If you refer back to the electromagnetic spectrum, you will note that the shorter wavelength rays posses more energy.
Importance of the Energy of Radiation
It is important to know the energy of the radiation because the energy controls the penetrating power of the radiation. Higher energy radiation can penetrate more amounts of material and harder to penetrate materials. This is important for a doctor taking x-rays so that there is not too much energy which can harm their patient. In NDT it's important so that we can properly penetrate the material or structure we want to inspect.
Measuring Radiation
It is common practice to measure x and gamma rays in one of two units, which are thousand electron volts (Kev), or million electron volts (Mev). What is an electron volt, you might ask? An electron volt is an amount of energy equal to the energy gained by one electron when it is accelerated by one volt. For example, if one electron was accelerated by a potential of 10 thousand volts (10 Kv), then the electron would have an energy of 10 thousand electron volts (10 Kev). If all of the accelerating energy were converted to electromagnetic energy, it would result in a 10 Kev x-ray.
Review:
- Radiation is an electromagnetic (EM) wave that has no charge and no mass.
- EM radiation can be characterized by frequency, wavelength, and energy.